
Features
Recirc
Assessing the toxicity of peracetic acid to early Atlantic salmon life-stages
January 26, 2023 By Natalie Redman, Freshwater Institute
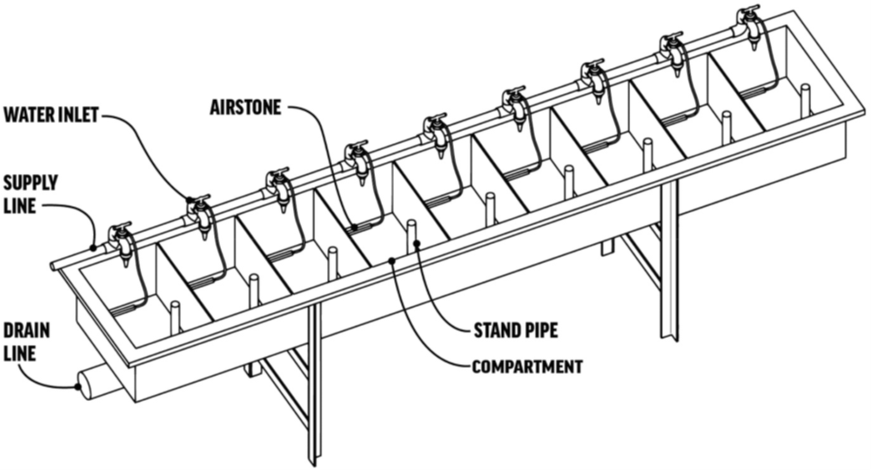 Illustration of challenge troughs utilized in fry and fingerling toxicity experiments. (Redman et al., 2022) (Image: Freshwater Institute)
Illustration of challenge troughs utilized in fry and fingerling toxicity experiments. (Redman et al., 2022) (Image: Freshwater Institute) Ubiquitous bacterial and fungal pathogens that reside in land-based recirculating aquaculture systems (RAS) and egg incubation systems can cause elevated mortality in juvenile fish populations under substandard environmental conditions. However, the availability of environmentally friendly, food-safe disinfectants that can be used to reduce these pathogens without harm to fish or RAS biofilters is limited.
While several approved disinfectants, such as hydrogen peroxide and ozone, have proven to be effective, the need for safer, low-cost alternatives would be welcomed by the land-based aquaculture industry. Peracetic acid (PAA), for example, is an antimicrobial disinfectant sold commercially as a stabilized mixture of acetic acid, hydrogen peroxide, and water. PAA is extremely active against microorganisms at low concentrations and it poses minimal threat to the environment, as it rapidly degrades in water and is not known to produce toxic by-products.
Due to its novelty for use in aquaculture applications, there has been minimal research regarding PAA’s applicability for use in RAS and egg incubation systems in the United States. Thus far, PAA has only been registered for surface disinfection by the US EPA when fish are absent from tanks. Therefore, an important step in assessing PAA’s suitability as an aquaculture disinfectant was to evaluate its toxicity to various fish life stages by determining its 24-hour LC50 value – the concentration at which approximately 50% mortality occurs within 24 hours.
The toxicity of PAA to early life stages of Atlantic salmon had not been assessed until The Conservation Fund’s Freshwater Institute (FI) conducted the research summarized herein. A comprehensive article describing this research was first published in the journal, Aquaculture Research (Redman et al., 2022).
Design and methodology
Three juvenile Atlantic salmon life stages were selected for the PAA toxicity experiments: eyed eggs, fry (0.16-0.18 g), and fingerlings (i.e., early parr; 16.3 ± 0.7 g), and a commercial PAA product VigorOx SP-15 (PeroxyChem) (15% PAA and 10% hydrogen peroxide) was selected to create the PAA doses that were tested at each life stage. To characterize the source water for each toxicity trial, samples were collected once before PAA application during each experiment. Subsequent water quality testing was carried out in FI’s Water and Environmental Chemistry Lab and at the USDA-ARS Stuttgart National Aquaculture Research Center. This research was reviewed and approved by FI’s Institutional Animal Care and Use Committee.
Atlantic salmon eggs
Disease-free-certified salmon eggs were received from a commercial supplier and maintained in heath tray stacks within an incubation system that recycled 99.8% of the water. Makeup water for the systems was sourced from a contained freshwater spring located on the property. Nine PAA treatments (0, 300, 400, 500, 600, 700, 800, 900 and 1000 mg/L PAA) were administered for 5 and 10 minutes each in 1L glass jars by diluting the PAA stock solution. Twenty eggs were added to each jar containing the treatment. Egg mortality was assessed 24 hours after the initial dose through visual observations of egg colour change (i.e., very pale orange or white) and via use of a salt flotation solution (105 g/L NaCl) technique as described by Leitritz and Lewis (1980).
Fry and fingerlings
Atlantic salmon fry were stocked in challenge trough compartments, with each trough divided into nine 35L sections containing an individual water inlet and double standpipe drain. The troughs received water pumped from a 9.5 m3 RAS containing a 5.3 m3 culture tank stocked with larger Atlantic salmon. Twenty fry were stocked into each trough compartment required for the experiment. The troughs were maintained as flow-through systems during a 24-hour acclimation period and then adjusted to static baths during PAA treatment periods.
Nine PAA treatments (0, 3.5, 4, 4.5, 5, 5.5, 6, 6.5 and 7 mg/L PAA) were tested in triplicate. Each compartment remained in a static bath state for 24 hours after the PAA treatment was administered. The LC50 value for fry (0.16-0.18g) was determined 24 hours after the initial dose via visual observations of mortality.
Atlantic salmon fingerlings (16.3 ± 0.7 g) were assessed using the same setup and procedure described above. Nine PAA treatments (0, 4.5, 4.75, 5, 5.25, 5.5, 5.75, 6.0, and 6.25 mg/L PAA) were tested in triplicate, and mortality was assessed after 24 hours.
Toxicity results
Toxicity Relationship Analysis Program (TRAP) version 1.30a and the Trimmed Spearman-Karber (Erickson, 2015; Hamilton et al., 1977; Hamilton et al., 1978) techniques were used to calculate LC50 values and the results of both were reported by Redman et al. (2022). Because the resulting LC50 values were similar between approaches, calculated values from the more accessible TRAP software are reported in this article.
After exposure for five minutes, no mortality was observed at lower PAA concentrations; however, no surviving eggs were observed in the 1000 mg/L PAA treatments. When eggs were exposed to various PAA concentrations for 10 mins, significant mortality occurred at lower concentrations and no surviving eggs were observed in the 800, 900, and 1000 mg/L PAA treatments. The determined LC50 values for the 5 and 10-min treatments were 781.5 ± 12.7 (mean ± SE) mg/L and 485.0 ± 12.3 mg/L PAA, respectively. The NOEC (no observed effect concentration), the concentration at which there were no discernable negative effects, was 500 and 300 mg/L PAA for the 5- and 10-minute egg treatment groups, respectively.
Twenty-four hours after various PAA treatments were administered, no surviving fry were observed in the 5.0, 5.5, 6.5, and 7.0 mg/L PAA treatment groups. The 5- and 10-minute LC50 values for fry were 4.0 ± 0.04 and 4.1 ± 0.10 mg/L PAA, respectively. Twenty-four hours after various PAA treatments were administered, no surviving fingerlings were observed in the 6.25 mg/L PAA treatment group. The LC50 values for fingerlings were 5.3 ± 0.03 and 5.3 ± 0.06 mg/L PAA, respectively. Mortality curves for all experiments are illustrated in Figure 1.
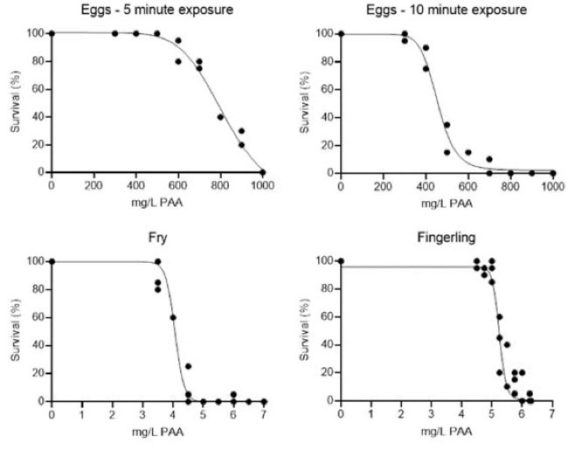
Figure 1. Early juvenile life-stage Atlantic salmon mortality curves during toxicity experiments: (i) eyed eggs, 5 min exposure (top left), (ii) eyed eggs, 10 min exposure (top right), (iii) fry stage (bottom left), and (iv) fingerling stage (bottom right). (Redman et al., 2022) (Image: Freshwater Institute)
Important water quality findings
Water quality profiles for each trial are shown in Figure 2. Research has shown that the degradation and disinfection efficacy of PAA is affected by water chemistry. For example, Liu et al. (2014) observed that higher DOC increases the rate of PAA degradation. Given that the present fry and fingerling toxicity trials were carried out during separate weeks, RAS water quality varied slightly between studies. For example, DOC was lower during the fingerling toxicity experiment compared to the fry toxicity study, which may have coincided with slightly slower PAA degradation and a slightly higher LC50 value for salmon fingerlings.
Additionally, the RAS nitrate-nitrogen concentration was higher during the fry toxicity experiment than it was during the fingerling toxicity experiment, and this difference may have influenced LC50 values. In addition to variations in water quality, the relative size differences between life stages likely contributed to increased PAA tolerance in fingerlings, resulting in higher LC50 values compared to fry.
Concentrations are expressed in mg/L unless otherwise noted.
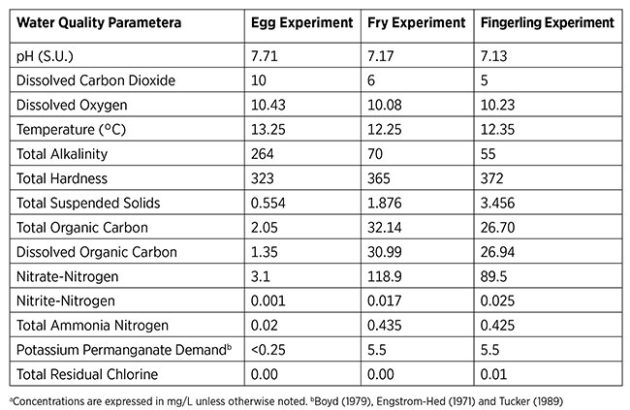
Figure 2. Water quality profiles for water used during the Atlantic salmon egg, fry and fingerling PAA toxicity experiments. (Redman et al., 2022) (Image: Freshwater Institute)
Implications for RAS
These findings support the development of PAA as a disinfectant that could potentially be used by the Atlantic salmon land-based RAS industry. It is important to note that the PAA concentrations that were deemed safe for Atlantic salmon fry and fingerlings may negatively impact beneficial nitrifying bacteria that thrive in RAS biofilters; therefore, additional research is required to understand the holistic effect of these PAA treatment doses in RAS.
In addition, future research should consider the limitations of this study, and should aim to include, among other things, consistent RAS water chemistry across experiments when expanding on these findings. Results of PAA application will vary based on RAS water chemistry profiles, and therefore it is imperative that aquaculturists consider their system’s specific water quality profile when developing PAA treatment regimes.
References
1. Erickson, R. J. (2015). Toxicity Relationship Analysis Program (TRAP), Ver 1.30a. EPA/600/C-11/ 002 U.S.
Environmental Protection Agency.
2. Hamilton, M.A., Russo, R.C., & Thurston, R.V. (1977). Trimmed spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environmental Science & Technology, 11, 714-719; correction 12,417 (1978).
3. Hamilton, M.A., Russo, R.C., & Thurston, R.V. (1978).
Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environmental Science Technology, 12, 417.
4. Leitritz, E., & Lewis, R. C. (1980). Trout and salmon culture: Hatchery methods (Vol. 164). UCANR Publications.
5. Liu, D., Steinberg, C. E., Straus, D. L., Pedersen, L. F., & Meinelt, T. (2014). Salinity, dissolved organic carbon and water hardness affect peracetic acid (PAA) degradation in aqueous solutions. Aquacultural Engineering, 60, 35–40.
6. Redman, Natalie, et al. “Assessing the toxicity of peracetic acid to early Atlantic salmon Salmo salar life-stages.” Aquaculture Research 53.14 (2022): 5097-5104.
Print this page
Advertisement
- Optimal hatching condition protocols on B. altianalis seed production in Uganda
- Commercial probiotics, enzymes in Pacific white shrimp trumps fermented probiotics





